Research at the Institute of Radical Chemistry focuses on compounds carrying unpaired electrons. We master their structure through synthesis, characterization and simulations to access a broad range of applications, from advanced materials to therapeutic uses.

🏆Congratulations to Prof. Laurence Charles, winner of the 2025 Confirmed Researcher Award from the Physical Chemistry Division of the French Chemical Society and the SCF-Sud PACA 2026 Great Prize from the French Chemical Society ! !! 👏👏👏

Florence Chaspoul is a chemical engineer in the SMBSO team. She began her career in the private sector before joining the university through a research contract. Since 2001, she has been working on projects related to analytical chemistry, particularly in the fields of environment and health. She values the diversity of the projects, the scientific curiosity they foster, and the training of students.
If, like her, you would like to join the staff of ICR and AMU, please consult AMU’s job offers.

Congratulations to Julie Broggi, researcher in the PCR team, who has been named one of the 2025 Junior Chair laureates of the Institut Universitaire de France (IUF)! 👏🎉
A well-deserved recognition of the excellence and rigor of her scientific career !!

The #holi of #science_XXelles! Between Giulia Mollica and powders, it’s a story of magnets 🧲. Discover the career of this CNRS research director and other profiles in the book “Girls Are Perfect for Science!” available for pre-order now!

ICR wraps up the year on a high note with its Annual General Meeting.
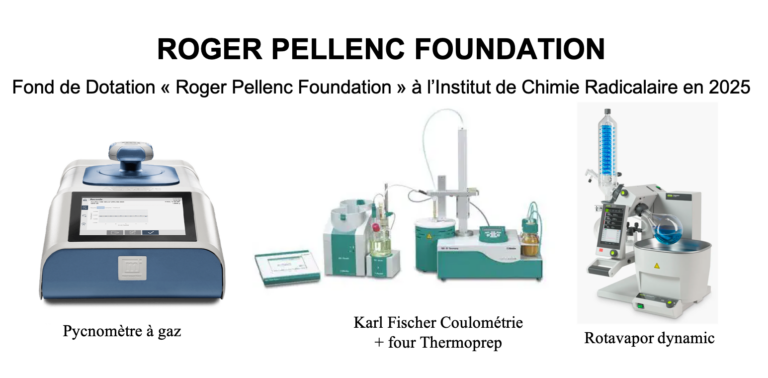
The CROPS team at the Institute of Radical Chemistry thanks the Roger PELLENC Foundation for supporting its research activities in the field of lithium metal batteries in 2025

From Vitrolles to Mirabeau-en-Luberon, and all the way to schools and college of Aix en Provence, Gardanne and Gréasque, our researchers shared their passion for chemistry with boundless enthusiasm!
Hands-on experiments, exciting demonstrations, and lively discussions helped spark curiosity among young budding scientists.
A wonderful opportunity to showcase research in a new light and inspire future vocations!
Special thanks to Béatrice Tuccio, Julie Broggi, Gérard Audran and Abdoulaye Sarr ! #FêtedelaScience
Congratulations to Dr Isaure Sergent, who received the 2025 AMU thesis prize for her work on the contribution of ion mobility spectrometry to the decoding of digital polymers. This thesis was supervised by Prof. Laurence Charles from the SACS team and Dr Anouk Siri from the CT team.


Novachim marked its 40th anniversary of innovation and commitment at the heart of the Chemistry & Materials industry. This anniversary year was dedicated to recognizing the players transforming the chemistry of tomorrow!
Among the highlights of the event, Camille Nguyen, doctoral student in the SMBSO team, shone by embodying the new generation of female talent during an inspiring panel discussion: “Chemistry is feminine!”
Meet Éric Besson, CMO team member, and member of the AMUtech interdisciplinary institute! Come ask questions, share ideas, and be amazed at the Festival of Sciences and Arts, organized by Aix-Marseille University.
📅 Join us on Thursday, September 18, from 3:30 p.m. to 5:00 p.m., at the Mucem Forum.


On July 1, 2025, the ICR gathered its staff on Frioul Island for a day of scientific lectures, a quiz, and a convivial dinner. It was a valuable occasion for exchange and sharing about the life of the institute. Many thanks to the organizing team!
The second edition of the Explore Festival—co-organized by Aix-Marseille University and CNRS Provence et Corse, in partnership with Inserm—features dozens of fun and interactive scientific activities open to everyone through Sunday. Hervé Clavier and Eric Besson will present their ANR-funded research to the general public at various locations throughout the city.
